STUDY PROBLEMS - Experiment #6 研究问题 - 实验6
These questions will not be graded; they are similar to possible quiz questions. The solutions to these and all other study problems are posted on Courseworks and can be discussed at office hours. 这些问题不会被评分;它们类似于可能的测验问题。这些以及所有其他学习问题的解答都发布在Courseworks上,可以在办公时间讨论。
Spectrochemical series: 光谱化学序列:
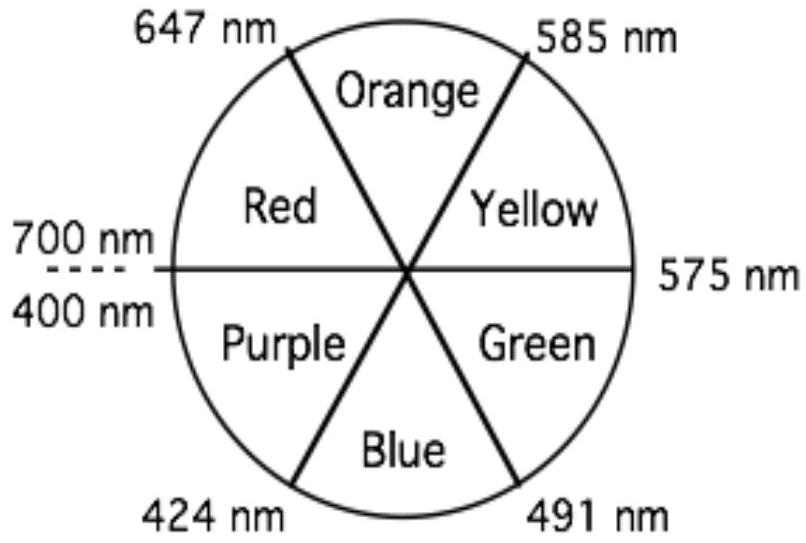
Q1 问题1
Ions such as and are called . (fill in the blank) 像和这样的离子被称为。(填空)
Q2 问题2
What is the coordination number of the central metal ion in ? 在中,中心金属离子的配位数是多少?
Q3 问题3
What is the coordination number of the central metal ion in ? 在中,中心金属离子的配位数是多少?
Q4 问题4
What is the oxidation state of iron in ? 在中,铁的氧化态是多少?
Q5 问题5
What is the oxidation state of cobalt in ? 在中,钴的氧化态是多少?
Q6 问题6
What are the possible geometries of a metal complex with a coordination number of six? A coordination number of four? 配位数为六的金属配合物可能具有哪些几何结构?配位数为四的呢?
Q7 问题7
In an octahedral complex, electrons in and orbitals experience a greater repulsion from the lone pairs of electrons on ligands because these orbitals . (fill in the blank) 在八面体配合物中,和轨道中的电子会感受到来自配体上孤对电子的更大排斥力,因为这些轨道 。(填空)
Q8 问题8
Which of the following complexes will not have a color in solution? Why?
以下哪些配合物在溶液中不会呈现颜色?为什么?
Q9 问题9
How would transition metal ions be classified using the Lewis definitions of acids and bases? Generally, what must a ligand have to bond to a metal? What do we mean when we say that a bond is a coordinate covalent bond or dative bond? 如何根据路易斯酸碱理论对过渡金属离子进行分类?通常,配体必须具备什么条件才能与金属形成键?当我们说键是配位共价键或配位键时,我们指的是什么?
Q10 问题10
Rank the following complex ions in order of increasing wavelength of light absorbed.
按吸收光波长增加的顺序排列以下配合物离子。
Q11 问题11
The following test tubes each contain a different chromium complex ion. 以下试管中分别含有不同的铬配合物离子。
Tube 1 is purple, Tube 2 is yellow, and Tube 3 is green 试管1是紫色的,试管2是黄色的,试管3是绿色的
For each compound, predict the predominant color of light absorbed. If the complex ions are , and , what is the identity of the complex in each test tube? 对于每种化合物,预测吸收光的主要颜色。如果配合物离子是、和,那么每个试管中的配合物的身份是什么?
In Beran lab manual:
Prelaboratory Assignment from Experiment #36: all Transition Metal Complexes 过渡金属配合物
Q1 问题1
Consider the coordination compound, . Use the definitions in the Introduction to identify the following with the formula and charge (if applicable). 考虑配位化合物。使用引言中的定义来识别以下内容,包括化学式和电荷(如果适用)。 a. the ligand(s) a. 配体 b. the complex b. 配合物 c. the coordination sphere c. 配位层 d. the coordination number of cobalt d. 钴的配位数
Q2 问题2
Write the formula of the complex ion that forms between 写出以下之间形成的配合物离子的化学式 a. the ligand and the platinum(II) ion with a coordination number of four. a. 配体和铂(II)离子,配位数为四。 b. the ligand ethylenediamine (abbreviated "en"), and the chromium(III) ion with a coordination number of six. b. 配体乙二胺(缩写为"en")和铬(III)离子,配位数为六。 c. the ligand dihydrogen ethylenediaminetetraacetate, (abbreviated ), and the zinc(II) ion with a coordination number of four. See Table 36.1. c. 配体二氢乙二胺四乙酸(缩写为)和锌(II)离子,配位数为四。参见表36.1。
Q3 问题3
Experimental Procedure, Parts A-D. Identify the chemicals that are cited as cautions. 实验步骤,A-D部分。识别被列为注意事项的化学品。
Q4 问题4
Experimental Procedure, Parts A-D. Of the four ligands in the study, which is or are polydentate(s)? 实验步骤,A-D部分。在研究中的四种配体中,哪些是多齿配体?
Q5 问题5
Experimental Procedure, Parts A-D. The stability for a number of transition metal complexes is determined in this experiment. What is the chemical test that is used? Explain. 实验步骤,A-D部分。本实验确定了多种过渡金属配合物的稳定性。使用了什么化学测试?请解释。
Q6 问题6
Ammonia, , is a stronger ligand than but weaker than . Complete the balanced equations for the reactions that are predicted to occur. 氨是比更强但比更弱的配体。完成预测会发生的反应的平衡方程式。
Q7 问题7
Experimental Procedure, Part E. A sample of (molar mass ) is dissolved in deionized water. If an excess of ammonia is added to the solution, solid (molar mass ) forms. What is the theoretical yield of product from the reaction? 实验步骤,E部分。将的(摩尔质量)样品溶解在去离子水中。如果向溶液中加入过量的氨,会形成固体(摩尔质量 )。该反应的理论产量是多少?
Q8 问题8
When 6 M NaOH is slowly added to a solution containing chromium(III) ion, a precipitate forms. However when an excess of 6 M NaOH is added, the precipitate dissolves, forming a complex ion with a coordination number of four. a. Write the formula of the precipitate. b. Write the formula of the complex ion (for example, see Experiment 15).
当缓慢向含有铬(III)离子的溶液中加入6 M NaOH时,会形成沉淀。然而,当加入过量的6 M NaOH时,沉淀溶解,形成配位数为四的配合物离子。
a. 写出沉淀的化学式。
b. 写出配合物离子的化学式(例如,参见实验15)。
Q9 问题9
Compare the stability of an ammonia complex and ethylenediaminetetracetate, complex with the addition of the hydroxide ion, . 比较氨配合物和乙二胺四乙酸配合物在加入氢氧根离子时的稳定性。
Laboratory Questions from Experiment #36: 1-5, 7
Q1 问题1
Part A.2. Is the chloride ion or water a stronger ligand? Explain. A.2部分。氯离子和水,哪个是更强的配体?请解释。
Q2 问题2
Part B.1. Is water or ammonia a stronger ligand. Explain. B.1部分。水和氨,哪个是更强的配体?请解释。
Q3 问题3
Part B.2. Is ammonia or ethylenediamine a stronger ligand? Explain. B.2部分。氨和乙二胺,哪个是更强的配体?请解释。
Q4 问题4
Parts A-D. Of the five ligands- , and -studied in this experiment, which ligand appeared to be the strongest ligand? Why? Which ligand appeared to be the weakest? Why? A-D部分。在本实验中研究的五种配体- 和中,哪种配体似乎是最强的配体?为什么?哪种配体似乎是最弱的?为什么?
Q5 问题5
Parts A-D. Along period 4 of the periodic table, cobalt, nickel, and copper appear in succession. From your data, does a trend in the stability of complexes that they form seem to exist? Explain. A-D部分。在周期表的第四周期中,钴、镍和铜依次出现。根据你的数据,它们形成的配合物的稳定性是否存在趋势?请解释。
Q7 问题7
Part E.2. Identify the precipitate that forms before the addition of excess conc . Hint: Ammonia is a base. E.2部分。识别在加入过量的浓之前形成的沉淀。提示:氨是一种碱。